Introduction
Energy is defined simply by scientists as the capacity for doing work. Matter is the material (atoms and molecules) that constructs things on the Earth and in the Universe. Albert Einstein suggested early in this century that energy and matter are related to each other at the atomic level. Einstein theorized that it should be possible to convert matter into energy. From Einstein's theories, scientists were able to harness the energy of matter beginning in the 1940s through nuclear fission. The most spectacular example of this process is a nuclear explosion from an atomic bomb. A more peaceful example of our use of this fact of nature is the production of electricity from controlled fission reactions in nuclear reactors. Einstein also suggested that it should be possible to transform energy into matter.
Energy and matter are also associated to each other at much larger scales of nature. Later on in this chapter, we will examine how solar radiation provides the energy to create the matter that makes up organisms. Organisms then use some of this matter to power their metabolism.
Types of Energy
Energy comes in a variety of forms. The simplest definition of the types of energy suggests that two forms exist: kinetic energy and potential energy. Kinetic energy is the energy due to motion. A rock falling from a cliff, a bee in flight, wind blowing leaves of trees, and water following over a waterfall are all examples of kinetic energy. Potential energy is the energy stored by an object that can be potentially transformed into another form of energy. Water stored behind a dam, the chemical energy of the food we consume, and the gasoline that we putting in our cars are all examples of potential energy. Conversion of this energy occurs when the energy in food is used by an organism to energize its metabolism, when the water in the dam flows through turbines to produce electricity from motion, and when the gasoline is used in a engine to produce motion from combustion.
Some other forms of energy include heat, electricity, sound, energy of chemical reactions, magnetic attraction, energy of atomic reactions, and light. Definitions for a few of these types of energy are as follows:
Radiation - is the emission of energy from a material object in the form of electromagnetic waves and photons.
Chemical Energy - is the energy produced or consumed in chemical reactions.
Atomic Energy - is the energy released from an atomic nucleus because of a change in its subatomic mass.
Electrical Energy - is the energy produced from the force between two objects having the physical property of electrical charge.
Heat Energy - is a form of energy created by the combined internal motion of atoms in a substance.
On Earth, there are fundamentally only three ways in which energy can be transferred from one place to another: conduction, convection, and radiation. Conduction involves the adjacent transfer of heat energy from one atom to another through the mass of a gas, liquid, or solid. Condution results in the continuous flow of heat energy along a temperature gradient from areas of higher to lower temperature. Convection involves the transfer of heat energy by way of mass movements of a substance in gas or liquid form in a vertical direction (horizontal transfer is called advection). Convection is often seen as rising masses of gas or liquid called convection currents. It is important to note that energy transfer by way of conduction and convection depends on the presence of matter. These forms of energy transfer do not operate in the vacuum of space. Radiation is the only means of energy transfer that can occur across outer space. The transfer of radiation produced at the Sun's surface through space supplies the Earth with most of its energy.
Matter: Elements and Compounds
Matter is the material that makes up things in the Universe. All matter on the Earth is constructed of elements (see WebElements for the periodic table of elements). Chemists have described approximately 115 different elements. Each of these elements have distinct chemical characteristics. Table 6a-1 lists some of the chemical characteristics for 48 common elements found in the Earth's continental crust.
The smallest particle that exhibits the unique chemical characteristics of an element is known as an atom. Atoms are composed of yet smaller particles known as protons, neutrons, and electrons. A proton is a subatomic particle that has significant mass and contributes a single positive electrical charge to an atom. Neutrons also have significant mass but no electrical charge. Electrons are extremely light subatomic particles having a mass that is 1/1840 of a proton. Each electron also has a negative electrical charge.
Protons and neutrons make up the nucleus of an atom. As a result, most of an atom's mass is concentrated in the nucleus. Because protons are positively charged the nucleus has a positive charge equal to the number of these subatomic particles. Electrons are found orbiting outside the nucleus at various distances based on their energy level. The area occupied by the electrons has a negative charge equal to the number of these subatomic particles. If an atom has an equal number of electrons and protons its net electrical charge is zero. If there are more electrons than protons the charge of the atom is negative. Likewise, if there are less electrons than protons the charge of the atom is positive. In both cases, the exact charge is determined by subtracting protons from electrons. As a result, 4 protons minus 6 electrons give an atomic charge of -2.
The number of protons found in the nuclei of the different types of elements is unique and is referred to as the atomic number (Table 6a-1). All atoms of a specific element have the same number of protons in their nuclei. Atomic mass number is an atom's total number of neutrons and protons. Many elements have unequal numbers of neutrons and protons in their nucleus. An element's atomic weight refers to the total weight of neutrons, protons, and electrons. For example, the atomic weight of aluminum is 26.98 (Table 6a-1). Atomic number describes the number of protons found in an atom. For example, silver has an atomic number of 47 or 47 protons in its atom (Table 6a-1). Some elements can have variants containing different numbers of neutrons but similar numbers of protons. We call these variants isotopes. Carbon has two isotopes. Its most common form is carbon-12 which has 6 protons plus 6 neutrons. About 99% of the carbon on our planet is of this type. The isotope carbon-13 has 6 protons plus 7 neutrons. Carbon-14 is the rarest isotope of carbon containing 8 neutrons. Some isotopes are unstable and their nucleus tends to lose subatomic particles forming an element with a lower atomic mass. This process is known as radioactive decay.
| Table 6a-1: Characteristics of some of the common chemical elements found in the Earth's continental crust. |
| Element |
Chemical Symbol |
Atomic Number |
Common Atomic Mass Number |
Atomic Weight |
Percent in Continental Crust |
Required for all Life |
Required for Some Lifeforms |
Element Type |
Moderately Toxic |
Extremely Toxic |
| Aluminum |
Al |
13 |
27 |
26.98 |
8.2300 |
- |
X |
Metalloid |
- |
- |
| Antimony |
Sb |
51 |
122 |
121.75 |
0.00002 |
- |
- |
Metalloid |
- |
- |
| Arsenic |
As |
33 |
75 |
74.92 |
0.00018 |
- |
- |
Metalloid |
- |
X |
| Barium |
Ba |
56 |
137 |
137.34 |
0.0425 |
- |
- |
Metal |
- |
- |
| Beryllium |
Be |
4 |
10 |
9.01 |
0.00028 |
- |
- |
Metal |
- |
X |
| Bismuth |
Bi |
83 |
209 |
208.98 |
0.000017 |
- |
- |
Metal |
- |
- |
| Boron |
B |
5 |
11 |
10.81 |
0.0010 |
- |
- |
Metalloid |
- |
- |
| Bromine |
Br |
35 |
80 |
79.91 |
0.00025 |
- |
- |
Nonmetal |
- |
- |
| Cadmium |
Cd |
48 |
112 |
112.40 |
0.00002 |
- |
- |
Metal |
- |
X |
| Calcium |
Ca |
20 |
40 |
40.08 |
4.1000 |
X |
- |
Metal |
- |
- |
| Carbon |
C |
6 |
12 |
12.01 |
0.0200 |
X |
- |
Nonmetal |
- |
- |
| Chlorine |
Cl |
17 |
35.5 |
35.45 |
0.0130 |
- |
X |
Nonmetal |
X |
- |
| Chromium |
Cr |
24 |
52 |
52.00 |
0.0100 |
- |
- |
Metal |
X |
- |
| Cobalt |
Co |
27 |
59 |
58.93 |
0.0025 |
- |
X |
Metal |
- |
- |
| Copper |
Cu |
29 |
63.5 |
63.54 |
0.0055 |
X |
- |
Metal |
X |
- |
| Fluorine |
F |
9 |
19 |
19.00 |
0.0625 |
- |
X |
Nonmetal |
X |
- |
| Gallium |
Ga |
31 |
70 |
69.72 |
0.0015 |
- |
- |
Metal |
- |
- |
| Germanium |
Ge |
32 |
73 |
72.59 |
0.00015 |
- |
- |
Metalloid |
- |
- |
| Gold |
Au |
79 |
197 |
196.97 |
0.0000004 |
- |
- |
Metal |
- |
- |
| Hydrogen |
H |
1 |
1 |
1.008 |
1.4000 |
X |
- |
Nonmetal |
- |
- |
| Iodine |
I |
53 |
127 |
126.90 |
0.00005 |
- |
X |
Nonmetal |
- |
- |
| Iron |
Fe |
26 |
56 |
55.85 |
5.6000 |
X |
- |
Metal |
- |
- |
| Lead |
Pb |
82 |
207 |
207.19 |
0.00125 |
- |
- |
Metal |
- |
X |
| Lithium |
Li |
3 |
6 |
6.94 |
0.0020 |
- |
- |
Metal |
- |
- |
| Magnesium |
Mg |
12 |
24 |
24.31 |
2.3000 |
X |
- |
Metal |
- |
- |
| Manganese |
Mn |
25 |
55 |
54.94 |
0.0950 |
X |
- |
Metal |
- |
- |
| Mercury |
Hg |
80 |
201 |
200.59 |
0.000008 |
- |
- |
Metal |
- |
X |
| Molybdenum |
Mo |
42 |
96 |
95.94 |
0.00015 |
X |
- |
Metal |
- |
- |
| Nickel |
Ni |
28 |
59 |
58.71 |
0.0075 |
- |
- |
Metal |
- |
X |
| Nitrogen |
N |
7 |
14 |
14.01 |
0.0020 |
X |
- |
Nonmetal |
- |
- |
| Oxygen |
O |
8 |
16 |
16.00 |
46.4000 |
X |
- |
Nonmetal |
- |
- |
| Palladium |
Pd |
46 |
106 |
106.40 |
0.000001 |
- |
- |
Metal |
X |
- |
| Phosphorus |
P |
15 |
31 |
30.97 |
0.1050 |
X |
- |
Nonmetal |
- |
- |
| Platinum |
Pt |
78 |
195 |
195.09 |
0.0000005 |
- |
- |
Metal |
- |
- |
| Potassium |
K |
19 |
39 |
39.10 |
2.1000 |
X |
- |
Metal |
- |
- |
| Rubidium |
Rb |
37 |
85.5 |
85.47 |
0.0090 |
- |
- |
Metal |
- |
- |
| Selenium |
Se |
34 |
79 |
78.96 |
0.000005 |
- |
X |
Nonmetal |
X |
- |
| Silicon |
Si |
14 |
28 |
28.09 |
28.2000 |
- |
- |
Metalloid |
- |
- |
| Silver |
Ag |
47 |
108 |
107.87 |
0.000007 |
- |
- |
Metal |
- |
X |
| Sodium |
Na |
11 |
23 |
22.99 |
2.4000 |
- |
X |
Metal |
- |
- |
| Sulfur |
S |
16 |
32 |
32.06 |
0.0260 |
X |
- |
Nonmetal |
- |
- |
| Thorium |
Th |
90 |
232 |
232.04 |
0.00096 |
- |
- |
- |
- |
- |
| Tin |
Sn |
50 |
119 |
118.69 |
0.00020 |
- |
- |
Metal |
X |
- |
| Titanium |
Ti |
22 |
48 |
47.90 |
0.5700 |
- |
- |
Metal |
- |
- |
| Tungsten |
W |
74 |
184 |
183.85 |
0.00015 |
- |
- |
Metal |
- |
- |
| Uranium |
U |
92 |
238 |
238.03 |
0.00027 |
- |
- |
- |
- |
- |
| Vanadium |
V |
23 |
51 |
50.94 |
0.0135 |
- |
X |
Metal |
X |
- |
| Zinc |
Zn |
30 |
65 |
65.37 |
0.0070 |
X |
- |
Metal |
- |
- |
Elements can be classified as being either metals, nonmetals, or metalloids (Table 6a-1). Metals are elements that usually conduct heat and electricity and are shiny. Nonmetals do not conduct electricity that well and are normally not shiny. Metalloids have characteristics that are in between metals and nonmetals.
Elements with a net positive or negative charge are called ions. Chemists indicate the number of positive or negative charges on an ion using a superscript after the element's symbol. For example, calcium has two positive charges and is written as Ca2+. Some common negatively charged ions include nitrate (NO3-), sulfate (SO42-), and phosphate (PO43-).
Positive and negative ions are electrically attracted to each other. This mutual attraction allows for the bonding of atoms to occur forming structures of matter that are larger than just one atom. When similar atoms bond together they construct molecules. Atoms of different elements joined together form compounds (Figure 6a-1). Sodium chloride (or table salt), is an ionic compound consisting of sodium (Na+) and chloride (Cl-). In nature, it forms as a three-dimensional array of oppositely charged ions (Figure 6a-2). Many of the Earth's substances have a molecular structure similar to sodium chloride.
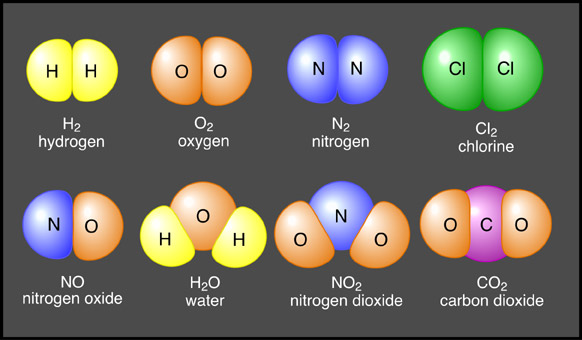
| Figure 6a-1: Some common molecules and compounds. The molecules in the top row bond with each other by sharing electrons. The compounds in the bottom row also share electrons. However, these joins are called ionic bonds. |
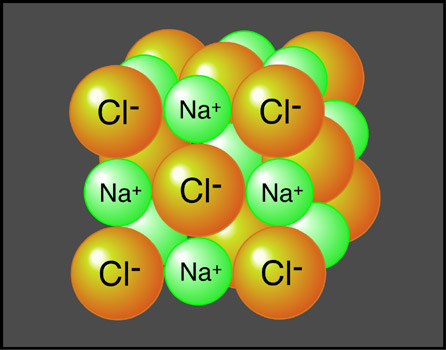
| Figure 6a-2: Atomic representation of sodium chloride or table salt. This compound forms in nature as a highly ordered, three-dimensional network of oppositely charged ions. The bonds that form between the sodium (Na+) and (Cl-) chloride ions give this compound great internal strength allowing it to form large crystals. |
Inorganic vs. Organic
Compounds and molecules constructed in living tissues are commonly called organic. Forms of matter not formed by living things are termed inorganic. Organisms like autotrophs usually create organic matter by consuming inorganic molecules and compounds from the lithosphere, hydrosphere, and atmosphere. An example of an autotroph is any photosynthesizing plant. Heterotrophs consume and assimilate other living things to create their organic matter. Herbivores and carnivores are examples of heterotrophs.
There are four general categories of organic compounds: lipids, carbohydrates, proteins, and nucleic acids.
Lipids - are composed of carbon atoms that have two hydrogen atoms attached. Lipids are commonly known as fats and oils, and belong to the family of molecules known as hydrocarbons.
Carbohydrates - are composed of carbon, oxygen, and hydrogen atoms. Some examples are sugars, starch, and cellulose.
Proteins - are organic compounds that are made primarily of carbon, hydrogen, nitrogen, and some other minor elements that are arranged into 20 different compounds known as amino acids.
Nucleic Acids - are composed primarily of different combinations of carbon, hydrogen, nitrogen, oxygen, and phosphorus. They are very complex compounds being created by the atomic linking of thousands of individual atoms. DNA or deoxyribonucleic acid, the genetic blueprint of life, is an example of a nucleic acid.
Cells
All organisms are composed of one or more of cells. Cells are the smallest self-functioning unit found in living organisms. Cells are also where the processes of metabolism and heredity occur in an organism. Cells arise by the cellular division of a previously existing cell. Biologists have differentiated two basic types of cells in organisms. Bacteria, archaea, and cyanobacteria have cells that are quite uncomplicated in terms of structure and function. Quite simply, they lack internal organization. These cells are commonly known as prokaryotes (Figure 6a-3).
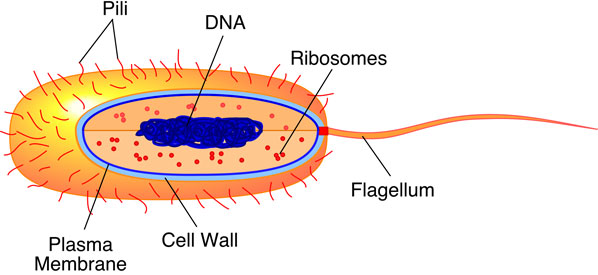
| Figure 6a-3: Typical prokaryote cell. These cells are about 1 to 10 micrometers in size. The cell is encased by rigid cell wall and a plasma membrane. Within the cell, the two most obvious structures are ribosomes and DNA. The DNA is not bounded by a membrane. Many prokaryote cells also have a flagellum which is used for movement. |
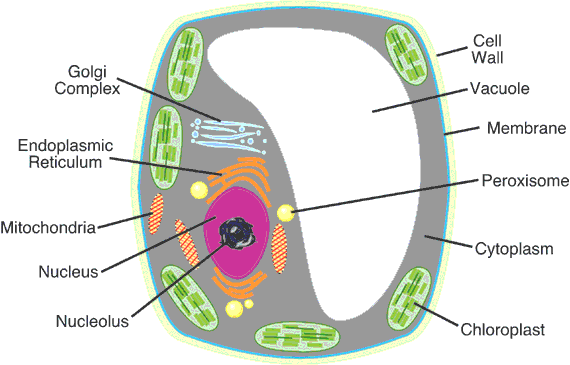
The cells of plants and animals are more complex than those of bacteria, archaea, and cyanobacteria. We call these type of cells eukaryotes. Eukaryotic cells have a membrane enclosed nucleus which contains the organism's DNA. Plant and animal cells also contain a variety of membrane-bound structures known as organelles. Figures 6a-4 and 6a-5 describe the various structures found in typical plant and animals cells.
Eukaryotic cells also show great variation in terms of size. White blood cells of mammals are some of the smallest eukaryotic cells with a diameter between 3 to 4 micrometers. Ostrich ovum are very large cells with a diameter of about 100 micrometers.
|
Figure
6a-4: Typical plant cell. Plants
cells differ from animal cells in the following
ways: they have a cell wall, chloroplasts,
and they often contain a large central vacuole. |
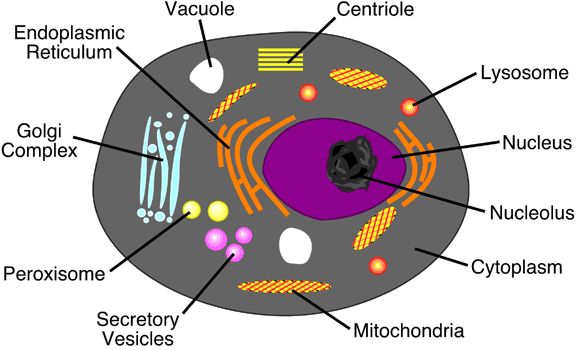
|
Figure
6a-5: Typical animal cell. |
Internally, cells contain specialized structures known as organelles that carry out distinct cellular functions. Within these structures enzymes, a type of protein, are used to facilitate and regulate various chemical reactions. Table 6a-2 describes the function of a variety of cell structures including many organelles.
|
Table
6a-2: Description and function of
common cell structures. |
| Structure | Description | Function |
| Cell Wall | Outer layer on a cell composed of cellulose or other complex carbohydrates. | Helps to support and protect the cell. |
| Plasma Membrane | A layer composed of lipids and proteins that controls the permeability of the cell to water and dissolved substances. | Regulates the movement of material into and out of the cell. |
| Flagellum (Flagella pl.) | Threadlike organelle that extends from the surface of the cell. Found in both prokaryotes and eukaryotes. | Used for movement of the cell or to move fluids over the cell's surface for absorption. |
| Pilus (Pili pl.) | Hollow, hairlike structures made of protein found on prokaryote cells. | Allows cell to attach itself to another cell. |
| Ribosomes | Tiny, complex structures composed of protein and RNA. Often attached to endoplasmic reticulum. | Ribosomes are involved in protein synthesis. |
| Endoplasmic Reticulum | Extensive system of internal membranes. | Forms compartments to isolate cell substances. |
| Nucleus | Double membrane structure that encases chromosomes. | Cell structure which directs protein synthesis and cell reproduction. |
| Chromosomes | Long strands of DNA. | Store hereditary information. |
| Nucleolus | Aggregations of rRNA and ribosomal proteins. | Area were ribosomes are manufactured. |
| Golgi Complex | Flattened stacks of membranes. | Used in the collection, packaging, and distribution of synthesized molecules. |
| Peroxisomes | Membrane confined spherical body about 0.2 to 0.5 micrometers in diameter. | Formed by the endoplasmic reticulum. Converts fats into carbohydrates. Detoxifies potentially harmful oxidants. |
| Lysosomes | Membrane confined spherical body about 0.2 to 0.5 micrometers in diameter. | Formed by the golgi complex. Contains digestive enzymes for braking down old cellular components. |
| Centrioles | Long hollow tubes composed of protein. Not found in plant cells. | Influence cell shape, move chromosomes during reproductive division, and are the internal structure for flagellum. |
| Secretory Vesicles | Membrane enclosed sack created at the golgi complex. | These structures contain cell secretions, like hormones and neurotransmitters. The secretory vesicles are then transported to the cell surface where they are release to the environment outside the cell. |
| Vacuole | Voids within the cytoplasm. Quite large in plant cells. | Used to store water and waste products. |
| Cytoplasm | Semifluid mixture that occupies most of the cell's interior. Contains sugars, amino acids, and proteins. Also, contains a protein fiber network. | Medium in which organelles and other internal structures exist in. Fiber network support the shape of the cell and anchor organelles to fixed positions. |
| Mitochondria | Elongated structures about 1 to 3 micrometers long. Resemble aerobic bacteria. | Structure which converts sugar into energy through oxidation. |
| Chloroplasts | Elongated structures with vesicles containing chlorophyll. | Site of photosynthesis. |
Cells can also be classified according to how they obtain their energy. Some cells have the ability to use light or chemical energy found in the outside environment to manufacture their own sugars, fats, and proteins. We call these types of cells autotrophs. All 400,000 species of plants and a few species of bacteria use sunlight and the process of photosynthesis to obtain their energy. Some bacteria breakdown molecules found in the environment to release chemical energy to sustain their life. Organisms can also obtain their energy by consuming other organisms. These organisms are called heterotrophs. Heterotrophs include most types of bacteria and all of the animal and fungi species.
Some organisms consist of just one cell (bacteria, algae, and protozoa). However, most organisms are multi-cellular. Within multi-cellular organisms, groups of cells can become specialized to carry out a certain function. We call these functional groups of cells a tissue. An organ is a structure composed of several different types of tissues. Organs also have a specific structure and a particular function.